RAPID COVID-19 IgM/IgG Antibody Test Kit
DISCOVID-branded COVID-19 IgM/IgG Antibody Test Kit is discontinued under the policy stated in Section IV.D of the FDA’s Policy for Coronavirus Disease-2019 Tests.
DISCOVID has been evaluated at the Clinical Microbiology Laboratories, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, with 93% sensitivity and 95.9% specificity in patients that have symptom onset ≥ 7 days.
DISCOVID is also being evaluated by National Institutes of Health’s National Cancer Institute (NIH/NCI).
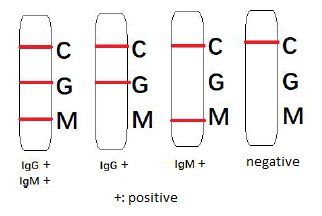
In response to the quick detection of SARS-CoV-2 infection, we developed a rapid yet simple kit, DISCOVID, that takes <5 minutes to get the results. DISCOVID detects the presence of COVID-19 disease-specific IgM and IgG antibodies in a small drop of patient’s blood. The result of the test can be read/recorded without the need for expensive equipment during the test.
COVID-19 IgM/IgG Antibody Test Kit
(Colloidal Gold)
DISCOVID
INTENDED USE & LIMITATIONS
- DISCOVID is used by CLIA laboratories for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human serum, plasma or venous whole blood specimens suspected of having COVID-19.
- For professional in vitro diagnostic use only, and not for home testing.
- Test results from DISCOVID should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status, and must be considered with other clinical information available to the physicians.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- DISCOVID is not for the screening of donated blood.
- The reference value of serological antibody detection is limited for the immune-compromised patients or patients who receive immunosuppressive therapy.
- Use appropriate personal protective equipment when collecting and handling the specimens.
- Optimal results may be obtained when starting to test >7-10 days after symptom onset. If symptom persists while no or weak G/M band appears, repeat the test in 2-3 days.
- For Emergency Use Authorization (EUA) Only.